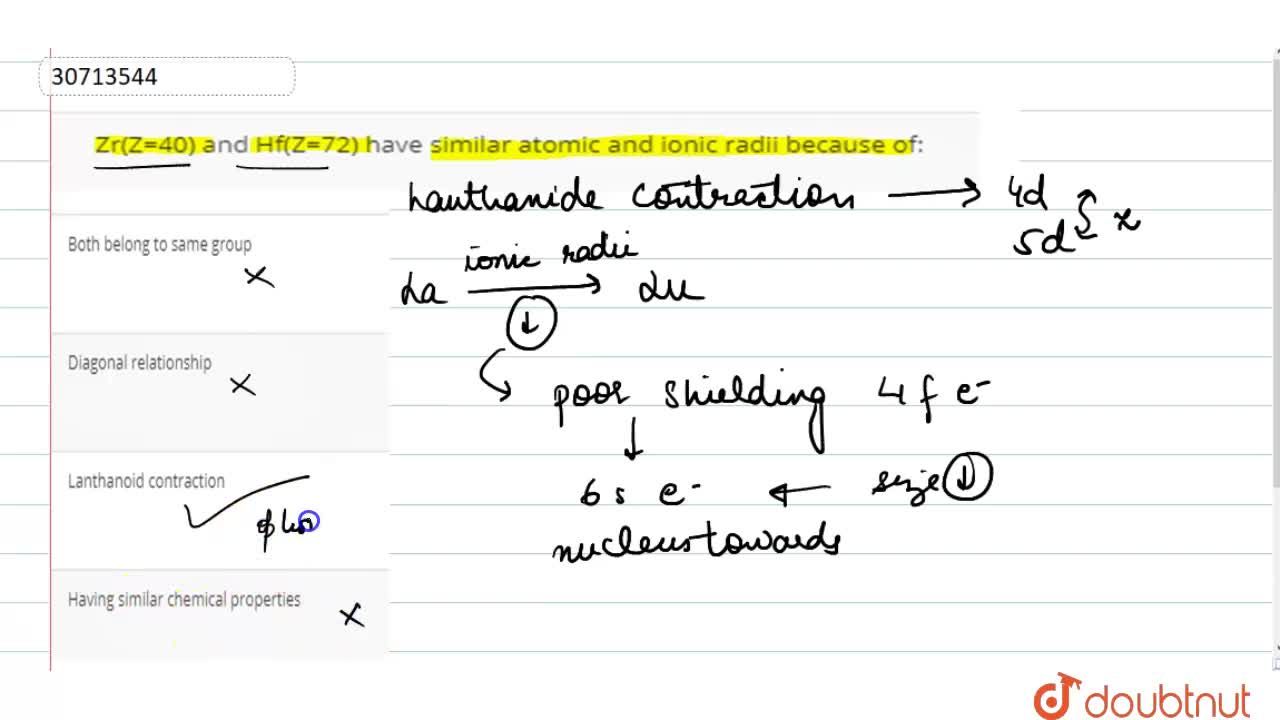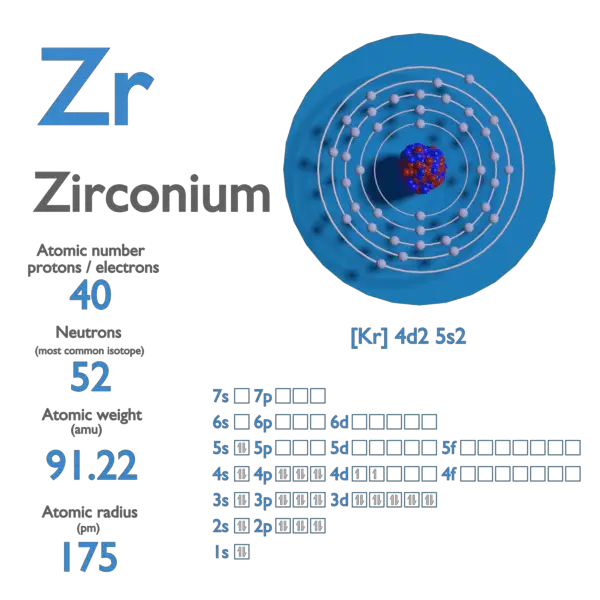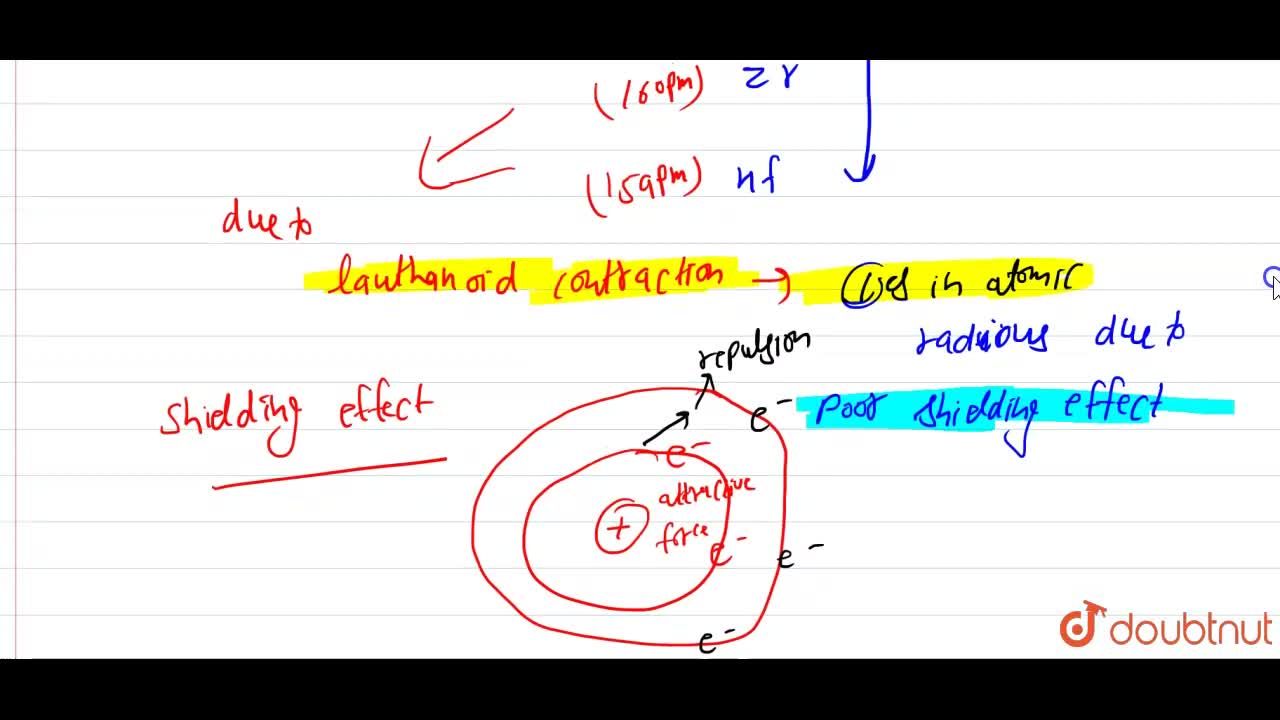
Similarity in the radius of Zr and Hf is explained on the basis of:A.Lanthanide contractionB.Inert pair effectC.Same outer shell configurationD.Anomalous configuration

Chemistry. D and F Block - 3 Session Session Objectives 1.f-Block elements 2.Introduction to lanthanides 3.Oxidation state 4.lanthanide contraction 5.Chemical. - ppt download

Design and fabrication of Ti–Zr-Hf-Cr-Mo and Ti–Zr-Hf-Co-Cr-Mo high-entropy alloys as metallic biomaterials - ScienceDirect

Change in atomic radius as a function of the period in the periodic... | Download Scientific Diagram
SOLVED:Zr and Hf have almost equal atomic and ionic radii because: (a) of diagonal relationship (b) of lanthanide contraction (c) of actinide contraction (d) hoth ho ane e

Comparison of Ti, Zr, and Hf as Cations for Metallocene-Catalyzed Olefin Polymerization | Organometallics

DFT insights into the electronic structure, mechanical behaviour, lattice dynamics and defect processes in the first Sc-based MAX phase Sc2SnC | Scientific Reports